文章
Mechanical Evaluation of Suture Anchors for Soft Tissue Repair

By Engaged Expert
Maciej Jakucki.
When you think about medical implants, knee or hip replacements typically come to mind. However, there are various categories of implants, like those dedicated to repairing bone or soft tissue related conditions. Suture anchors are implants used to repair soft tissue including muscles, ligaments, tendons, and skin.
它是相对常见的听到肌肉strain or a torn ligament, and these injuries often result in severe pain, coupled with long term implications if untreated. Whether related to athletic events, or general trauma due to falls or injury, surgical assistance is often needed to repair the damage and improve quality of life. Grafts, sutures, bone anchors, and other devices are used to reshape or repair the injury and restore functionality. As a result of the increased occurrences of trauma, bone and suture anchors have been readily accepted in surgical procedures to repair soft tissue injuries.
随着人口老龄化和参与体育活动的参与,预计未来10年将在未来10年内继续大幅生长。
This article will focus on the different methods of suture anchor testing used to evaluate the mechanical performance of design components.
骨骼和缝线锚的类型
The three most common categories are general suture anchors made of traditional materials (e.g., PEEK), nitinol suture anchors, and absorbable suture anchors. They come in a variety of configurations, such as fully threaded, knotless, or push to lock, and are intended for many applications:
- Upper extremity: rotator cuff, scapholunate, UCL (ulnar collatoral ligament), biceps, and wrist flexor/extensor repairs
- 下肢:achilles破裂,脚/脚踝不稳定,Quadriceps修理
指导文件
For bone anchor evaluation, there are two main FDA guidance documents that outline the requirements for these devices:
指导文件概述了提交的策略并概述了设备描述和分类,谓词比较,生物相容性,无菌,再处理,热原,保质期和包装,MRI兼容性,非临床表现测试,临床表现测试和标签的建议。
There are several published testing methods as well as recommendations outlined in the FDA Guidance, and the method selection depends on the unique design features of the devices. Ultimately, any testing strategy will have to mitigate the observed risks of the device. For example, the fixation, eyelet design and suture must be evaluated. Clinically, common failure modes include loss of fixation in the bone, suture failure through the eyelet or suture failure through the tissue. The interface of the eyelet and the passed-through suture requires additional consideration, particularly as new suture materials are being evaluated.
Sampling
应在最终包装条件下评估灭菌的装置(骨锚和缝合线)。没有最低可接受的样本量,但每次指导普遍接受五(5)的样本量。应依赖于观察到的数据变异性或减轻其他风险并提供统计有效结果的其他样本。
Test methodologies
Dependent on the material of the bone anchor and suture, the relevant test environment should be chosen. For absorbable or nitinol materials, it is recommended to test in saline solution and appropriate temperatures, although other solutions may be used. The FDA Guidance lays out the test methods, but ASTM F564 is commonly also referenced as the methodologies are similar, even though the specification is focused on bone staples.
Suture characterization:最常用的方法涉及测试缝合线的拉伸强度。每个样本安装在测试夹具中,捆绑或夹持,如手术技术推荐的,并以恒定速率拉动失效。该测试迅速评估缝合线和预期用途的基线强度。
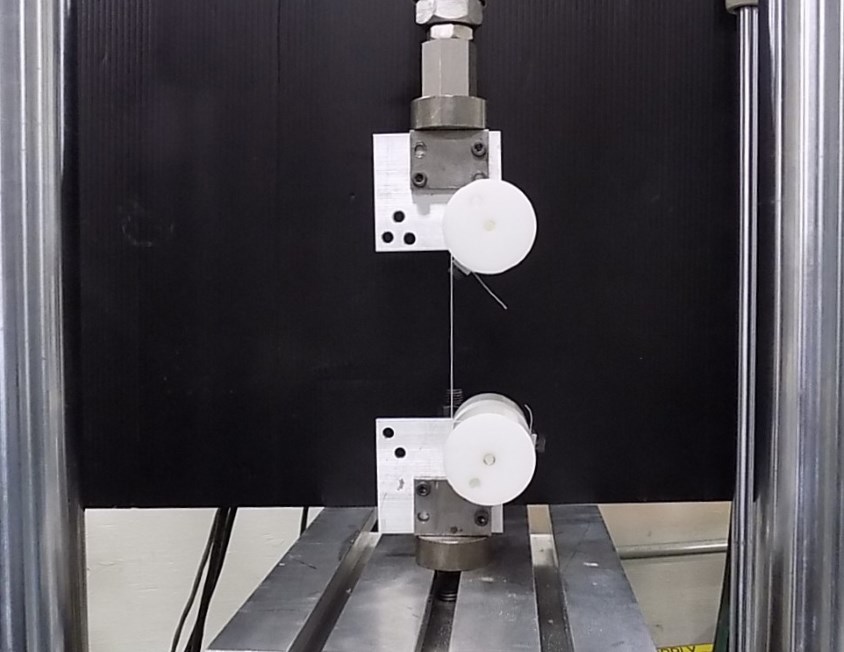
Bone anchor insertion testing:The bone anchors are inserted into a bone substitute that has equivalent density to that of the anatomically intended insertion location. The bone substitute should conform toASTM F1839 - Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments。可以钻取导向孔,这些孔依赖于手术技术,然后将骨锚插入预期深度,测量扭矩或力。ASTM F543通常引用骨螺钉。
Pullout and interconnection testing:该测试可以复制三种常见的缝合锚式故障模式:锚/骨接口,锚/缝合连接或缝合线本身。一旦将骨锚连接到每个上述方法中,将恒定的拉伸载荷施加到缝合线上并拉到故障。这可能导致三种临床失败模式中的任何一种,并将分离最弱的组件。另外,可以设计测试方法和夹具以在缝合线,孔眼/缝合界面或锚部中强制失效,从而可以独立地评估每个锚点。如果骨架由组件组成,则应执行测试以评估互连机构。
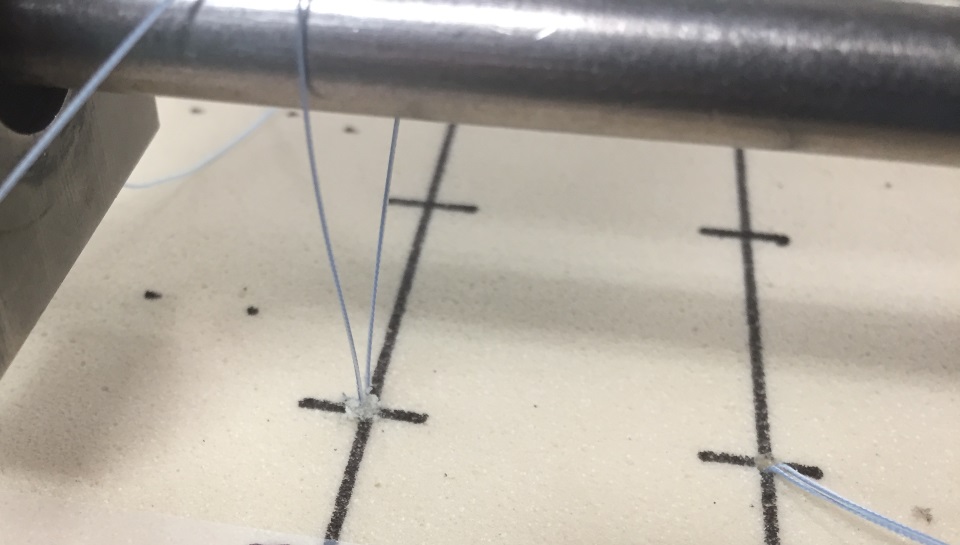
Fatigue testing:The above test methods evaluate the static strength in the different loading modalities; however, fatigue or cyclic testing should also be performed to evaluate how the device performs over repetitive loading. There are several methods and parameters to consider when performing fatigue testing:
- Predicate performance – first and foremost, the method performed should match the identified predicate test method so that the results can be compared. Bone anchors tend to be more readily available as predicate devices, and it is common
- 环路数量和打结方法
- 负载角度施加 - 0°,45°和90°是用于评估的共同角度。
- 锚旋转角度
- 预负载 - 应适当地张紧测试组件以模拟松弛载荷
- 频率 - 由于许多设备是聚合物,测试频率不应超过5 Hz,它们通常运行较慢以最小化磨损效果。
- Test cycles – 500 or 10,000 sinusoidal cycles are a common target cycle count but should be evaluated independently for relevance.
- 应选择负载 - 负载以提供足够的比较。
在完成疲劳测试后,如果在目标循环计数之前未发生缝合锚锚,则应执行静态拉出测试以评估前后疲劳性结果。
Creep testing:对于可吸收装置,可以在装载下发生蠕变。在适当的流体环境中进行测试,并且施加静电保持负载。应在保持负载的时间长度上监视位移。
Degradation testing:The FDA Guidance recommends multiple time points for evaluation as degradable materials will lose their structural and mechanical strength overt time. There are several methods for evaluating the levels of degradation:
- ASTM F1635 - Standard Test Method for in vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants
- ASTM F2502 - Standard Specification and Test Methods for Absorbable Plates and Screws for Internal Fixation Implant
Conclusion
While this overview looks specifically at the test methodologies to evaluate suture bone anchors, many of them can be used in other soft tissue applications. The methods are intended to evaluate the performance of each feature and attempt to replicate suture anchor failure modes. As new developments in the soft tissue repair market are introduced, these suture anchor test methods may be modified and improved, but will continue to serve as the basis for evaluating these types of devices.
For more information about our medical device testing services,contact our experts今天。
近190年的肯定
More from Element

Services
骨板骨螺钉和固定装置
We provide mechanical testing for bone plates, screws and fixation devices to ensure they function safely when needed most.
阅读更多

Services
脚,脚踝和肩部更换植入物测试
With a wide range of services, we are a single source provider for foot, ankle, extremity and shoulder implant testing.
阅读更多

Resource
医疗设备的测试协议
议定书和计划将减轻您的风险,防止混淆,设置明确的期望,并保留未来参考和使用的必要信息。
阅读更多

sector
Medical Device
作为一个全面的测试合作伙伴,您将享受单个供应商来源的福利,从机械测试,环境模拟到EMC和无线设备测试。
阅读更多